UNIT 7: MATTER AND FORCES
Solutions:
1. a) volume; b) density; c) buoyancy; d) density; e) metal
2. a) gravity; b) magnetism; c) buoyancy; d) friction; e) buoyancy; f) friction; g) gravity; h) friction
3. a) homogeneous, b) heterogeneous, c) heterogeneous, d) heterogeneous, e) heterogeneous, f) homogeneous, g) homogeneous, h) heterogeneous,i) heterogeneous, j) homogeneous, k) heterogeneous, l) homogeneous
4. a) P; b) C; c) P; d) C; e) P
FRIDAY June 12th:
Solutions:
1. Nylon: raincoats, backpacks, clothes
Plastic: pens, bags, toys
Polystyrene: food trays, packaging and computer and TV casing.
2. It breaks down or decomposes naturally.
Biodegradable: orange peel. It depends on the paper. Napkins will but magazines won’t. Batteries, plastic bag and glass won’t.
3. Look for the biodegradable symbol on the bags.
4. You can’t recycle ceramics, mirror glass or light bulbs. However, there are special recycling centres.
In your notebook 📓:
👉 Do the diagram of the unit.
👉Page 107: do activities 1, 2, 3 and 4.
TUESDAY June 9th:
1. a) volume; b) density; c) buoyancy; d) density; e) metal
2. a) gravity; b) magnetism; c) buoyancy; d) friction; e) buoyancy; f) friction; g) gravity; h) friction
3. a) homogeneous, b) heterogeneous, c) heterogeneous, d) heterogeneous, e) heterogeneous, f) homogeneous, g) homogeneous, h) heterogeneous,i) heterogeneous, j) homogeneous, k) heterogeneous, l) homogeneous
4. a) P; b) C; c) P; d) C; e) P
👉La tarea de hoy os la envío también por el Dojo
FRIDAY June 12th:
Solutions:
1. Nylon: raincoats, backpacks, clothes
Plastic: pens, bags, toys
Polystyrene: food trays, packaging and computer and TV casing.
2. It breaks down or decomposes naturally.
Biodegradable: orange peel. It depends on the paper. Napkins will but magazines won’t. Batteries, plastic bag and glass won’t.
3. Look for the biodegradable symbol on the bags.
4. You can’t recycle ceramics, mirror glass or light bulbs. However, there are special recycling centres.
REVIEW
In your notebook 📓:
👉 Do the diagram of the unit.
👉Page 107: do activities 1, 2, 3 and 4.
TUESDAY June 9th:
Solutions:
8. a. 1 b. 3 c. 2 d. 3 e. 2 f. 1 g. 4
11. Picture:(top) gravity(bottom) buoyant force
a. gravity
b. buoyant force
c. gravity
d. buoyant force
 Non-stick ceramic coating tolerates heat better, doesn’t peel off and doesn’t release harmful chemicals into the food we eat.
Non-stick ceramic coating tolerates heat better, doesn’t peel off and doesn’t release harmful chemicals into the food we eat.
FRIDAY June 5th:
Solutions:
2. PS: gold, iron, oxygen M: sea water, chocolate, soft drink
3. a) evaporation; b) filtration; c) sieving
4. Dissolving: sugar, salt, coffee powder Non-dissolving: rice, metal, plastic
9. Your own ideas

8. a. 1 b. 3 c. 2 d. 3 e. 2 f. 1 g. 4
11. Picture:(top) gravity(bottom) buoyant force
a. gravity
b. buoyant force
c. gravity
d. buoyant force
A NEW WORLD OF MATERIALS p.102
Nearly everything we use is made of materials
that have been created or modified by scientists and engineers to make
them perform better.
Man-made materials have transformed our everyday
life.
👉 Alloys
Mixing two or more elements, one of which must be a metal.
🎦 Watch the video
Brass,
for example, is an alloy of copper and zinc.
 | |||
| Copper |
 |
| Zinc |
Steel (iron+coal) is one of the most commonly used alloys in everyday life.
 |
| Coal |
 |
| Iron |
 |
| Steel |
👉 Improving your health
Surgeons can replace hip and knee joints with artificial joints made of a special ceramic materia.
A ceramic material is also used by dentists to repair teeth which have developed cavities.
👉New Materials in everyday life
 Non-stick ceramic coating tolerates heat better, doesn’t peel off and doesn’t release harmful chemicals into the food we eat.
Non-stick ceramic coating tolerates heat better, doesn’t peel off and doesn’t release harmful chemicals into the food we eat.
Polar fleece fabric is a
type of material that can be manufactured from recycled plastic bottles.
(Up to 150 pieces of clothing can be made from 3.700 large plastic
bottles).
👉Recent breakthrough
Concrete Cloth is a durable
and flexible waterproof building material made of cement sandwiched
between fabric.
It can be molded into any shape when wet and hardens on
contact with air. It is useful during natural disasters as it can be
used to make emergency shelters.
 |
| Concrete canvas shelter |
🌎 Fragile world: Modern day alchemist p. 105
👉Read the texts.
In your notebook 📓, do activities 1, 2, 3 and 4
In your notebook 📓, do activities 1, 2, 3 and 4
👉VOCABULARY
Alloy: aleación (mezcla de dos sustancias, al menos una de ellas debe ser un metal)
Brass: latón
Copper: cobre
Zinc:zinc
Steel:acero
Improving:mejorando
Surgeons: cirujanos
Replace: sustituir
Artificial joint: articualción artificial
Cavities: caries
Non-stick ceramic coating: revestimiento cerámico antiadherente (que no se pega)
Polar fleece: forro polar
Breakthrough: avances
Concrete cloth: tela de hormigón
Sandwiched: intercalado
Sandwiched: intercalado
Shelter: refugio
Alchemist: alquimista
FRIDAY June 5th:
Solutions:
2. PS: gold, iron, oxygen M: sea water, chocolate, soft drink
3. a) evaporation; b) filtration; c) sieving
4. Dissolving: sugar, salt, coffee powder Non-dissolving: rice, metal, plastic
9. Your own ideas
FORCES p.100
A force is a push or pull
that acts on an object.
🎦Watch the video
We can’t see forces, but we can see and feel their effects.
Forces can make
things move, stop, speed up, slow down, or change direction.
Forces can also make things change shape.

Some forces act
from a distance.
These forces are called non-contact forces.
Other forces act
through physical contact.
These are called contact
forces.
🎦 Watch the video
👉 Non-contact forces
🎦Watch the video
👉Contact Forces
👉VOCABULARY
Force: fuerza
Push: empujar
Pull: tirar
Contact:contacto
Magnetismo: magnetismo
Gravity: gravedad
Friction.ficción
Rub: frotar
Parachute: paracaidas
Buoyancy: flotabilidad
Upward force: fuerza hacia arriba
Submerged: sumergido
Displaces: desplaza
📓In your notebook:
TUESDAY June 2nd:
Solutions:
3) a. volume
b. thermal conductivity
c. mass
d. hardness
e. density
f. solubility
TYPES OF MATTER p. 99
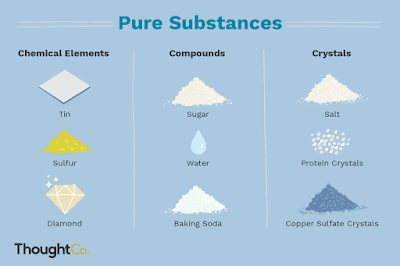
According to its composition, we can classify matter into pure substances and mixtures.
2. Mixtures are made up of two or more pure substances and can be homogeneous or heterogeneous.


- In a homogeneous mixture, such as the air we breathe or sea water, we cannot see the individual substances that make it up.
- In a heterogeneous mixture, such as sand or a salad, we can see the individual substances that make it up.
There are different methods to separate the substances in mixtures:Separate liquid from solid🎦Watch the videoSeparate two solids🎦 Watch the video
👉VOCABULARY
Pure substances: sustancias puras
Mixtures:mezclas
Heterogeneous: heterogenéneas (características diferentes)
Homogeneous: homogéneas (mismas características)
Filtration: filtración
Pour: echar
filter: filtro
Evaporation: evaporación
crystallise: cristalizado
Sieving: tamizar
📓In your notebook:
👉Page 99: do activities 2, 3 and 4.
👇 Activity 9
FRIDAY May 29th:
Solutions:
1) Gases: indefinite; container. Liquids: volume; shape. Solids: definite
2) (a) solid plane; liquid clouds; gas air. (b) solid volcano; liquid lava; gas. (c) solid plant; solid frost. (d) solid plant; liquid rain or dew. (e) solid trees; gas mist. (f) gas air; gas inside balloons; solid mountains and ground.
3) (1) b; (2) b; (3) a; (4) c
MATTER AND ITS PROPERTIES p. 98
🎦Watch the video
Matter is everything around us. Matter is made out of tiny particles called atoms. Some atoms join together to make groups known as molecules.
Apart from its colour, odour and taste, matter has other properties and can be found in three different states (solid, liquid and gas).
Matter: materia
Particles:partículas
Atoms:átomos
Molecules: moléculas
Odour: olor
Properties:propiedades, características
States: estados (sólido, líquido, gaseoso)
Volume:volumen (espacio que ocupa la materia)
Mass: masa (cantidad de materia en un objeto)
Density: densidad ( cantidad de masa en un determinado volumen)
Hardness: dureza
scratch-resistance: resistenica al rayado
Solubility:solubilidad (capacidad de una sustancia para disolverse en otra)
Dissolve: disolver
solution:solución (resultado de una disolución)
Thermal conductivity:conductividad del calor
Conductors:concuctor (que deja pasar el calor)
Insulators: aislante (que no deja pasar el calor)
Odour: olor
Properties:propiedades, características
States: estados (sólido, líquido, gaseoso)
Volume:volumen (espacio que ocupa la materia)
Mass: masa (cantidad de materia en un objeto)
Density: densidad ( cantidad de masa en un determinado volumen)
Hardness: dureza
scratch-resistance: resistenica al rayado
Solubility:solubilidad (capacidad de una sustancia para disolverse en otra)
Dissolve: disolver
solution:solución (resultado de una disolución)
Thermal conductivity:conductividad del calor
Conductors:concuctor (que deja pasar el calor)
Insulators: aislante (que no deja pasar el calor)
TUESDAY May 26th:
INTRODUCTION p. 94
The Universe is made up of matter and energy and empty space.
Everything around us, living and non-living,
is made up of matter: plants, animals, books, tables, the food we eat
and the water we drink. Even the air we breathe is made up of matter.
Matter can’t be created or destroyed; it just
changes from one state or form to another.
How many states of matter
are there in the Universe?
🎦Watch the video to remember
Identify the states of matter in the picture of the Titanic.
👉Page 96
📓In your notebook:
1) Label the diagram with the information about the three states of matter using the words in the box.
2) Look at the photos below and identify the three states of matter. Some photos may show more than one.
👉 Page 97
Remember: Matter can’t be created or destroyed; it just
changes from one form to another. How?
🎦Watch the video